Laboratories
ENERMAT Platform
Development of Materials and Processes for Energy Applications

Laboratories
Development of Materials and Processes for Energy Applications

The ENERMAT laboratory was established in 2014 through a collaboration between the Karlsruhe Institute of Technology (KIT) and EIFER. It is located at the Institute for Chemical Technology and Polymer Chemistry (ITCP) on the KIT Campus South
The key research areas include:

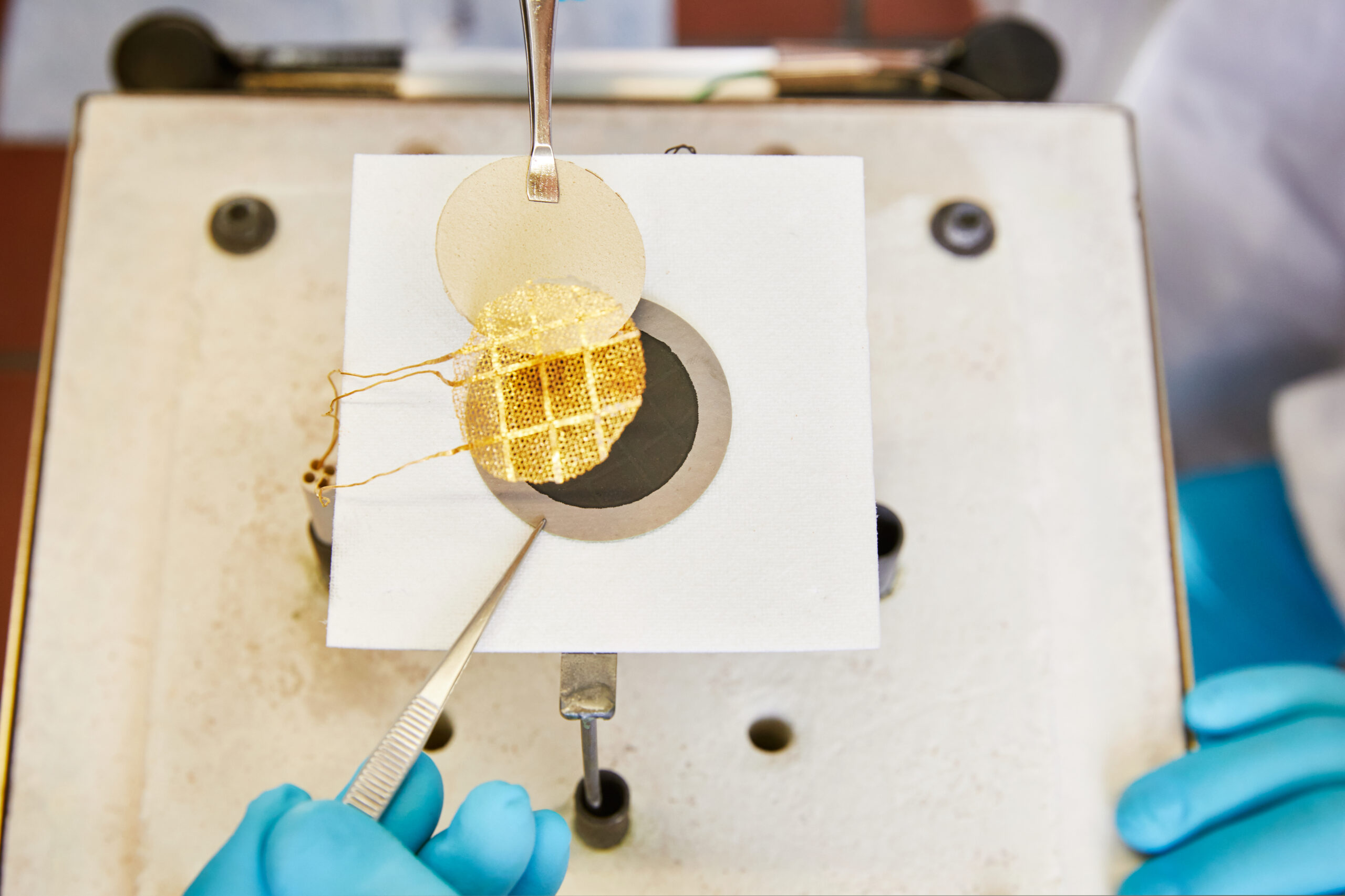
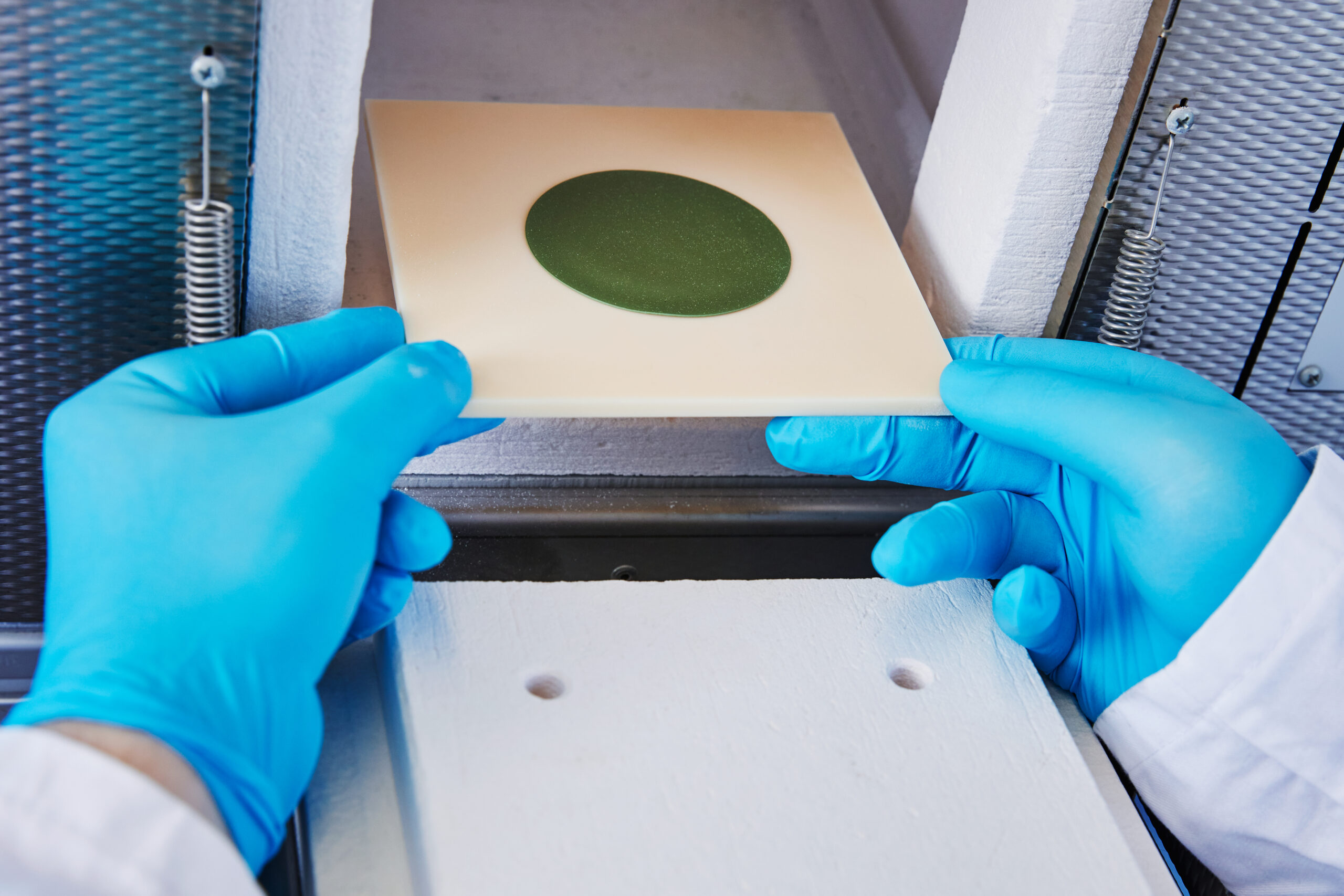
Manufacturing and characterization of innovative powder-metallurgical processed materials, covering the whole production process, from the raw material to the finished product in 3 steps.
ECOMET
HADES
PEPPER
Dr. Julian Dailly